hardness test of water by edta|complexometric titration with edta : manufacture Other Learning Activity (6) 174 Experimental Procedures Part A: Determination of total hardness 1. Pipette 50 cm3 mineral water into a conical flask. 2. Add 2 cm3 buffer solution followed by 3 drops of Eriochrome Black T indicator solution. 3. Titrate with 0.01 M EDTA until the solution turns from wine red to sky blue with The hexadecimal triplet #777888 definition is: Red = 119, Green = 120, Blue = 136 or CMYK: Cyan = 0.125, Magenta = 0.11764705882353, Yellow = 0, Black = 0.46666666666667. .
{plog:ftitle_list}
WEBJogo da raiz de ritmo rápido. Faça cartões exibindo um número com uma raiz quadrada fácil, como 9 (raiz quadrada: 3) e 16 (raiz quadrada: 4), evitando números como 6 (raiz quadrada: 2.449489.). Um estudante deve jogar em um momento e tentar encontrar o máximo de raízes quadradas que puder dentro de 25 segundos.

water hardness edta titration calculations
This SOP describes the procedure for measuring hardness by titration with standard EDTA solution to endpoint indicated by a color change. This method is based on Method 2340 C of . A major application of EDTA titration is testing the hardness of water, for which the method described is an official one. Hardness of water also can be tested by a more rapid test strip method. The commercial test strips contain EDTA and an indicator chemical to cause a color change when the calcium and magnesium in water react with the EDTA.Other Learning Activity (6) 174 Experimental Procedures Part A: Determination of total hardness 1. Pipette 50 cm3 mineral water into a conical flask. 2. Add 2 cm3 buffer solution followed by 3 drops of Eriochrome Black T indicator solution. 3. Titrate with 0.01 M EDTA until the solution turns from wine red to sky blue with 📏 Method 3: Hard Water Test Strips. Hard water testing strips offer a quick and easy way to test for hard water at home. A DIY water hardness test works by changing color to indicate the minerals present in the water. You .
This precipitate makes it hard to get suds to form and good detergent action in water high in Ca 2 + and Mg 2 +, hence the name “hard water”. A complexing molecule such as ethylenediaminetetraacetic acid (EDTA) is commonly used to determine the concentration of calcium and magnesium in natural water samples. A major application of EDTA titration is testing the hardness of water, for which the method described is an official one. Hardness of water also can be tested by a more rapid test strip method. The commercial test strips contain EDTA and an indicator chemical to cause a color change when the calcium and magnesium in water react with the EDTA.To determine the total hardness of the given samples by EDTA titrimetric method. Principle Originally, the hardness of water was understood to be a measure of the capacity of water for precipitating soap.
Water hardness can be measured using a titration with ethylenediaminetetraacetic acid (EDTA). At a pH of 10, calcium and magnesium ions form colorless, water soluble, complexes with EDTA. An idicator, known as a metal ion indicator, is required for the titration. The endpoint of the titration is when all the calcium and magnesium ions have been .Determine the hardness of water by EDTA titration and with Quantab® test strips. 16.2 EDTA TITRIMETRIC METHOD FOR TESTING HARDNESS OF WATER 16.2.1 inciple Pr of Method Ethylenediaminetetraacetic acid (EDTA) forms a Stable 1:1 complex with calcium or magnesium at pH 10. The metal ion indicators, calmagite and erio-Hardness of water is determined by titrating with a standard solution of ethylene diamine tetra acetic acid (EDTA) which is a complexing agent. Since EDTA is insoluble in water, the disodium salt of EDTA is taken for this experiment. EDTA can form four or six coordination bonds with a metal ion. Two type of hardness is present in water first is .Total water hardness is usually expressed as the milligrams of CaCO3 equivalent to the total amount of calcium and magnesium present in one liter of water (mg/liter, i.e., ppm). Water hardness may . resulting in a change in the concentration of .
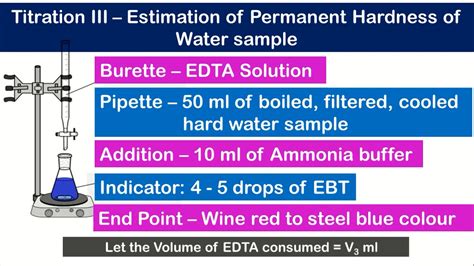
Hardness Test apparatus Requirements. Take 50 ml water sample; Conical flask 250 ml; Ammonia buffer solution: Dissolve 67.5 gm of ammonium chloride in 570 ml of ammonium hydroxide and dilute to one liter with deionised water. Eriochrome Black -T indicator: Dissolve 0.5 gm of EBT in 10 ml Methanol & makeup to 100 ml 0.02 N EDTA Solution: Take 3.723 gm of .This is the classic method to determine the total water hardness over a titration with EDTA solution.Patreon: https://www.patreon.com/randomexperimentsintern.The hardness of water is due in part to the presence of Ca2+ ions in water. The concentration of Ca2+ ions is usually expressed as ppm CaCO 3 in the water sample. This . EDTA itself is not very water soluble so the disodium salt is used, Na 2H 2C 10H 12N 2O 4. For the purpose of simplicity, Y will stand for C 10H 12N 2O 4. The EDTA we use is .
Temporary Hard Water. Temporary hard water is hard water that consists primarily of calcium (Ca 2 +) and bicarbonate (HCO 3-) ions.Heating causes the bicarbonate ion in temporary hard water to decompose into carbonate ion (CO 3 2-), carbon dioxide (CO 2), and water (H 2 O). The resultant carbonate ion (CO 3 2-) can then react with other ions in the .Calcium Analysis by EDTA Titration One of the factors that establish the quality of a water supply is its degree of hardness. The hardness of water is defined in terms of its content of calcium and magnesium ions. Since an analysis does not distinguish between Ca2+ and Mg2+, and since most hardness is caused by
4. Determination of Hardness • The hardness of water can be estimated by methods such as gravimetric analysis, EDTA titration, atomic absorption, etc., • In the above methods, EDTA titration is the most .
Ethylenediaminetetraacetic acid (EDTA), also called EDTA acid, is an aminopolycarboxylic acid with the formula [CH 2 N(CH 2 CO 2 H) 2] 2.This white, slightly water-soluble solid is widely used to bind to iron (Fe 2+ /Fe 3+) and .

Hard Water: Hard waters are generally considered to be those waters that require considerable amounts of soap to produce foam and that also produce scale in water pipes, heaters, boilers and . During the titration with EDTA, all free hardness ions are complexed as per Eq. 4a and subsequently, EDTA disrupts the wine red complex as it can form .
the 25 mL of the “hard water” sample should be measured with a 25-mL transfer pipet and the EDTA solution should be added from a buret. Preparation of Solutions Hard water sample. A hard water sample that mimics very hard water with approximately 1,000 ppm Ca2+ ion may be prepared by creating a slurry of 0.25 g of anhydrous CaCO 3 with 3 .Hardness of water is because of the presence of salts of calcium and magnesium. Know the different Types of Hardness in water and the process to Remove Temporary and Permanent Hardness. . Test Your Knowledge On Hardness Of Water Types And Removal! Q 5. Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz .Calcium hardness titration. The test for calcium hardness is very similar to the total hardness test. Traditionally, either murexide indicator (ammonium purpurate) or Eriochrome Blue-Black R indicator is followed by titration with EDTA. CalVer 2 Calcium Indicator has been developed by Hach to replace these indicators.Hard Water: Hard waters are generally considered to be those waters that require considerable amounts of soap to produce foam and that also produce scale in water pipes, heaters, boilers and . During the titration with EDTA, all free hardness ions are complexed as per Eq. 4a and subsequently, EDTA disrupts the wine red complex as it can form .
water "hardness" – the total calcium and magnesium content, typically expressed as equivalent . Test the pH of the solution using universal pH paper. Ideally, pH should be ≥ 10.3 (if not, consult . EDTA (M) Hardness (mg/L) NOTES 1) Standard operating procedure is to dry for one hour at 110 ºC. However, in a lab with many
hardness of water procedure
Water by Automatic Titration Key Words EDTA, complexometric, titrimetric, ASTM D1126, ASTM D511, ISO 6059, SM 2340C, SM 3500-Ca, magnesium, Orion 9720BNWP, Orion Star T930, Orion Star T940, titrator. Drinking water, process water, cooling water, boiler water, wastewater, surface water, environmental water, raw water. Introduction Total Hardness .The U.S. Geological Survey (www.usgs.gov) provides the following general guidelines for classification of waters: Soft: 0 to 60 mg/L hardness as CaCO3 Moderately hard: 61 to 120 mg/L hardness as CaCO3 Hard: 121 to 180 mg/L hardness as CaCO3 Very hard > 180 mg/L hardness as CaCO3 Both Ca2+ and Mg2+ can be determined by titration with .
Water by Automatic Titration Key Words EDTA, complexometric, titrimetric, ASTM D1126, ASTM D511, ISO 6059, SM 2340C, SM 3500-Ca, magnesium, Orion 9720BNWP, Orion Star T930, Orion Star T940, titrator. Drinking water, process water, cooling water, boiler water, wastewater, surface water, environmental water, raw water. Introduction Total Hardness .Collect about 75 cm 3 of soap solution in a small beaker.; Set up a burette and, using the small funnel, fill it with soap solution. Use a measuring cylinder to measure out 10 cm 3 of one of the samples of water from the list below into a conical flask: . Rain water (solution A)
Carton Puncture Tester distribute
Sporting e Benfica voltam a defrontar-se, desta vez para a primeira mão das meias-finais da Taça de Portugal 2023/24. Uma partida que pode acompanhar EM DIRETO em A BOLA. Leões e águias jogam .
hardness test of water by edta|complexometric titration with edta